Background Langerhans cell histiocytosis (LCH) is a rare, heterogeneous histiocytic disorder. We demonstrated liver involvement at baseline indicated poor survival in adult LCH in our previous study.
Aims The aim of this study was to describe clinical features, liver biochemistry, radiology findings, genomic analyses, treatment approaches, and outcomes of adult LCH patients with liver involvement.
Methods We conducted a retrospective analysis of all adult LCH patients (≥ 14 years) seen at Peking Union Medical College Hospital (Beijing, China) between January 2001 and December 2022. Liver involvement by one or more of the following: either hepatomegaly, defined as a liver edge greater than 3 cm below the costal margin at the mid clavicular line, or liver dysfunction defined either by abnormal serum biochemical tests including bilirubin greater than 1.5 times the upper limit of normal, alkaline phosphatase (ALP) greater than 1.5 times the upper limit of normal, γ-glutamyl transpeptidase (GGT) greater than 1.5 times the upper limit of normal; or histopathological findings of active disease.
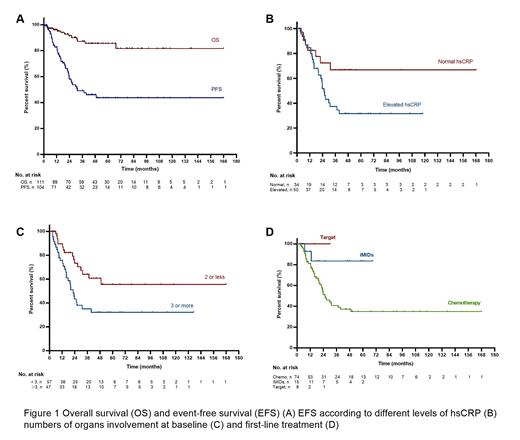
Results Among 451 newly diagnosed adult LCH patients, 93 patients had liver involvement at diagnosis and 22 patients had liver involvement at relapse were enrolled, including 80 males (69.6%), and the median age was 32 years (range, 15-66 years). 111 of 115 patients had full liver function test. 70 patients (63.1%) had elevated ALP; 96 patients (86.5%) had elevated GGT, and the median level was 157 U/L (range, 14-2249 U/L); 16 patients (14.4%) had elevated total bilirubin, and the median level was 12.5 umol/L (range, 1.7-248.1umol/L); Among 48 patients who had sufficient DNA for next-generation sequencing, BRAFV600E (20.8%), BRAFN486_P490 (37.5%) and MAP2K1(12.5%) were the most common alternations. In total, 99 patients (86.1%) received first-line systematic treatment, including 76 patients who received chemotherapies (methotrexate/cytarabine regimens, cytarabine monotherapy, vindesine/prednisone-based regimens, cladribine monotherapy and corticosteroid), 15 patients who received immunomodulatory drugs (IMiDs) therapies (thalidomide/cyclophosphamide/dexamethasone regimens and lenalidomide/dexamethasone regimens) and 8 patients who received target therapies (BRAF inhibitors and MEK inhibitors). After a median 39-month follow-up (range 1-168 months), thirteen patients died during follow-up. The estimated 3-year OS rate was 85.5%. 44 patients had disease progression. The median PFS duration was 31.4 months. Nine patients got cirrhosis during follow-up and 2 patients underwent liver transplantation. Univariate analysis was performed to evaluate the prognostic factors of OS and PFS. Patients with elevated total bilirubin at baseline had significantly shorter OS and PFS. Patients who had 3 or more abnormal serum live function tests (including ALT, AST, ALP, GGT, total bilirubin and ALB), and patients with elevated high sensitive C reactive protein (hsCRP) had significantly shorter PFS. Patients who received target therapy or iMIDs-based therapy had significantly longer PFS than patients who received other systemic treatments. In multivariable analyses, Patients who had 3 or more abnormal serum live function tests (HR 2.652, 95% CI 1.256-5.602), and treatment with iMIDs (HR 0.076, 95% CI 0.010-0.564) remained predictive factors for PFS.
Conclusion Adult LCH patients with liver involvement most had elevated GGT and ALP. Elevated hsCRP and 3 or more abnormal live function tests predict poor outcomes. Target therapy or iMIDs-based therapy showed better outcomes.
Disclosures
Goyal:Opna Bio: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal